Abstract
Background: Treatment of Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) has been revolutionized after the introduction of tyrosine kinase inhibitors (TKIs) to the backbone multi-agent chemotherapy regimens. While the majority of patients (pts) achieve complete remission (CR) following initial therapy, a significant proportion relapse. Here we examine the predictors of outcomes in pts with Ph+ ALL at first morphological relapse.
Methods: Clinical data from adult pts with Ph+ ALL who received frontline hyperCVAD chemotherapy with TKI at our institution between 4/2001 and 5/2018 were reviewed. Pts who had morphological relapse after first CR, defined as recurrence of BM blasts to ≥ 5% and/or development of central nervous system (CNS) or other extramedullary leukemia, were evaluated for predictors of second response and survival. Relapse free survival-2 (RFS2) was defined as the time from the date of second CR to the date of second relapse or death (censored at last f/u). Overall survival-2 (OS2) was defined as the time from the date of first relapse until death (censored at last f/u). The Kaplan Meier method was used to estimate RFS2 and OS2. Univariate and multivariate Cox proportional hazards models were used to determine associations between prognostic covariates and outcomes.
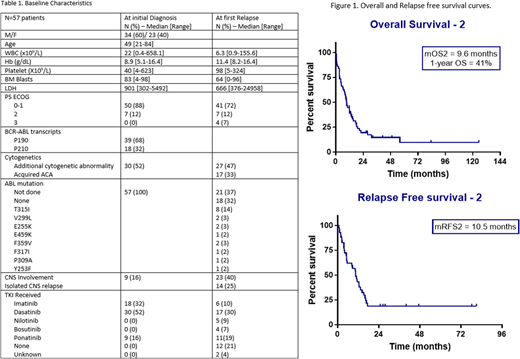
Results: We identified 233 pts with Ph+ ALL who received frontline hyperCVAD with TKI on clinical trials. 57 (25%) pts had morphological relapse after a median of 15.9 months (mo) from first CR (range: 5.3-94). Among these, only 5 pts had received an allogeneic hematopoietic cell transplantation (alloHCT) in first CR. Baseline characteristics of all pts are summarized in table 1. Salvage treatments for relapsed pts were re-challenge with hyperCVAD in 18 (32%) pts, other cytarabine-based regimens in 10 (18%) pts, asparaginase-based regimens in 7 (12%) pts, intrathecal chemotherapy alone in 6 (11%) pts, blinatumomab in 4 (7%) pts, and inotuzumab in 4 (7%) pts. 43 (75%) pts received a TKI in combination with their salvage treatment; of them, 19 (44%) continued with their initial TKI, while 24 (56%) pts changed to another TKI.
CR2 was achieved in 41 of 49 (84%) evaluable pts. 8 pts were not evaluable; early death in 4 pts or loss of f/u in the other 4 pts. Among pts achieving CR2, 23 pts were negative for minimal residual disease (MRD) by flow cytometry at time of CR2. 20 pts achieved major molecular response (MMR, defined as BCR-ABL PCR ratio of less than 0.1% IS) after a median of 2.2 mo (range, 0.5-5.1) from start of salvage treatment. 10 pts achieved complete molecular remission. The median number of cycles to CR2 was 1 cycle. 17 (30%) pts underwent alloHCT in CR2. Among the 41 pts achieving CR2, 22 had a second relapse after a median of 13.3 mo (range, 0.7-78). The median duration of CR2 in pts who had alloHCT was not reached versus 12.4 months (p=0.01) in pts who continued on maintenance chemotherapy. Median RFS2 was 10.5 mo (range, 0.2-81). 1-year and 2-year OS2 were 41% and 20% respectively (Figure 1). At last f/u, 47 (82%) pts had died; 28 (60%) died from disease progression, 7 (15%) from transplant related complications (GVHD,VOD), 4 (8%) from infections in CR, and 8 (17%) from other causes (such as myelodysplastic syndrome, motor vehicle accident). 10 pts are still alive after a median f/u of 30.4 mo, and are in continuous CR (7 pts in CR2, and 3 pts in CR3). On univariate analysis, patients with first remission duration of more than 12 mo had longer OS with a hazard ratio (HR) of 0.5 (95% CI, 0.28-0.92, p=0.03). Pts who relapsed with LDH ≥ 1200 U/L, or performance status of 2 or 3, or received either no TKI or imatinib had worse OS with a HR of 2.07 (95% CI, 1.06-4.05), 2.41 (95% CI, 1.17-4.96), and 3.82 (95% CI, 2-7.31), respectively. Achievement of MMR after salvage treatment was associated with better outcomes with HR of 0.39 (95% CI, 0.2-0.73) for OS2, and 0.43 (95% CI, 0.22-0.82) for RFS2 (p =0.01 for both). After adjusting for all other variables, LDH, achievement of MMR and the use of second or third generation TKI had a significant effect on OS.
Conclusion: The majority of pts with first relapsed Ph+ ALL will respond to subsequent salvage chemotherapy in combination with TKI. AlloHCT is feasible in CR2 and is associated with increased remission duration, but not survival. LDH less than 1200 at relapse, the use of second or third generation TKIs in salvage treatment and achievement of MMR predicted for improved survival.
Short:Takeda Oncology: Consultancy. Konopleva:Stemline Therapeutics: Research Funding. Jain:Astra Zeneca: Research Funding; Celgene: Research Funding; Pfizer: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding; Genentech: Research Funding; Abbvie: Research Funding; Servier: Research Funding; Cellectis: Research Funding; Seattle Genetics: Research Funding; ADC Therapeutics: Research Funding; Verastem: Research Funding; Servier: Research Funding; Incyte: Research Funding; Infinity: Research Funding; Incyte: Research Funding; Pharmacyclics: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Astra Zeneca: Research Funding; Seattle Genetics: Research Funding; BMS: Research Funding; Infinity: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Abbvie: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Verastem: Research Funding; Adaptive Biotechnologioes: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cortes:Daiichi Sankyo: Consultancy, Research Funding; Arog: Research Funding; Pfizer: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. O'Brien:Aptose Biosciences Inc.: Consultancy; Pfizer: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Sunesis: Consultancy, Research Funding; Janssen: Consultancy; Amgen: Consultancy; Pharmacyclics: Consultancy, Research Funding; Vaniam Group LLC: Consultancy; Celgene: Consultancy; Kite Pharma: Research Funding; Astellas: Consultancy; Regeneron: Research Funding; Abbvie: Consultancy; Acerta: Research Funding; Alexion: Consultancy. Kadia:Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Abbvie: Consultancy; Amgen: Consultancy, Research Funding; BMS: Research Funding; Jazz: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Consultancy; Abbvie: Consultancy; BMS: Research Funding; Celgene: Research Funding; Novartis: Consultancy; Celgene: Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy. Jabbour:Novartis: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Abbvie: Research Funding. Ravandi:Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Jazz: Honoraria; Sunesis: Honoraria; Abbvie: Research Funding; Jazz: Honoraria; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria; Sunesis: Honoraria; Abbvie: Research Funding; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria; Xencor: Research Funding; Xencor: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal